Limitations of DoE During Early-Stage Development of Dry Granulation Formulations
How to Avoid the Pitfalls of Design of Experiments in Roller Compaction
Being responsible for developing a dry granulate with a roller compactor, the formulator must face the challenge of performing first experiments with minimal amount of material and a maximum outcome of information. For this purpose, often a Design of Experiments (DoE) is created, especially as the FDA recommends using statistical tools.
How Do You Set Up a Design of Experiment?
The main parameters influencing the properties of a dry granulate are roll force, gap, and roll speed. Putting these parameters in a standard software for Design of Experiments, the output of a Box-Behnken design for example would be 15 trials with three equally spaced levels per parameter. In general, the extreme values of specific roll force, gap and roll speed are rarely used.
Drawbacks of DoE: What Do You Need to Know Before Performing a DoE?
Setting up such a Design of Experiment still requires lots of materials. Skilled operators, using a state-of-the-art lab-scale roller compactor, need at least 100 g of material per roller compaction trial, which sums up to 1.5–2.0 kg per formulation. This is too much for the early development, especially if more than one formulation is to be tested. Additionally, the evaluation of the granulate properties, especially performing force-hardness profiles, occupies much time for 15 different batches of granulate per formulation. The most important drawback by far is related to the properties of the formulation and its interaction with the roller compactor: the so-called manufacturing space.
Manufacturing Space for Roller Compaction
The manufacturing space describes the extent to which a roller compactor can process a formulation regardless of the final granule’s quality. Most formulations cannot be manufactured at any combination of specific roll force, gap, and roll speed. Draw-in issues are the most frequent cause of physical limitations. Also, too low bulk densities or too high densifications can be responsible for a narrowed manufacturing space.
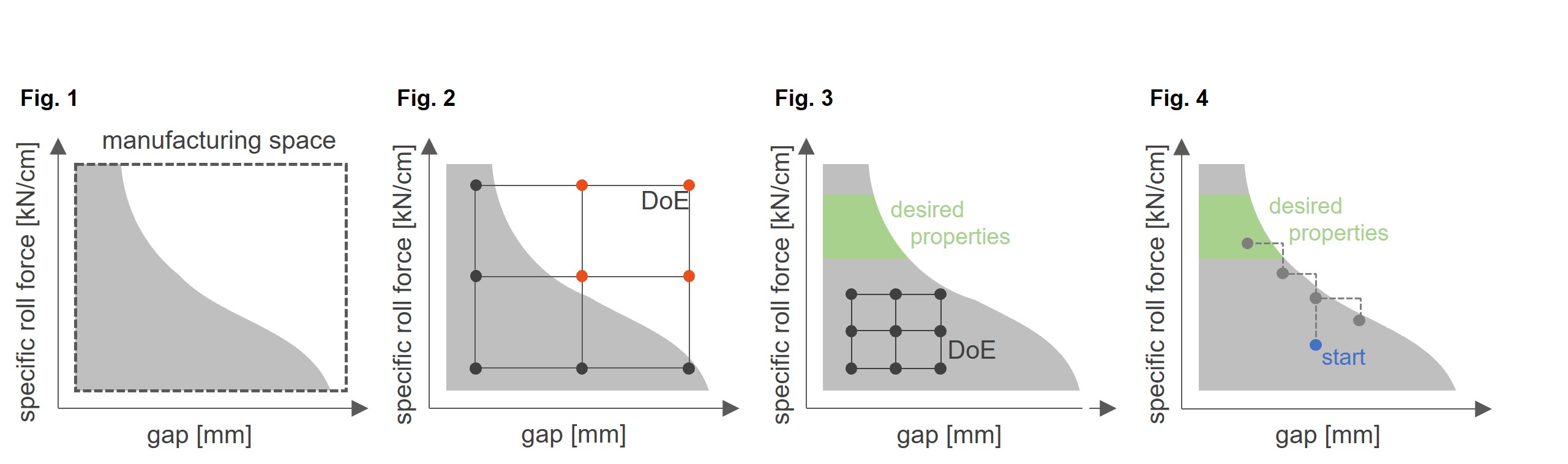
In Fig. 1 a simplified example for the interaction of specific roll force and gap are displayed. The dashed rectangle indicates the maximum possible manufacturing space. Only very few substances show such a wide manufacturing space. Most formulations are represented by the grey area, featuring draw-in issues at wider gaps. The result is that higher specific roll forces are simply not possible in roller compaction. The exact shape of a manufacturing space depends on the formulation as well as on machine parameters such as roll surface.
Design of Experiments vs. Manufacturing Space
From Fig. 2 it is obvious, setting up a Design of Experiments according to the specifications of the roller compactor without knowledge of the product is not meaningful as the red values are out of the manufacturing space. This eliminates or at least reduces the information value of the DoE. When adapting the DoE according to the maximum possible settings for force and gap, we might end up with the example shown in Fig. 3.
Although all DoE trials can be performed, the results are worthless because the DoE does not touch the green area in which the product can be manufactured with the desired properties. This leads to the conclusion that setting up a DoE without knowledge of the product and its interactions with the roller compactor is a waste of time and material. Exactly this knowledge is not available for the first experiments.
This situation shows the need of a different and more agile but still rational procedure to set up trials for a new formulation in roller compaction.
The Solution: Agile Testing Procedure for First Experiments in Roller Compaction
The Agile Testing Procedure is a good alternative to Design of Experiments. Based on the general rule for dry granulation—realizing as little densification as possible but as much as necessary—the Agile Testing Procedure starts at a medium gap and a rather low specific roll force (ref. to Fig 4). To save material, the roll speed is slow. If this setting is possible the next trial is performed at a higher force to obtain more densification. As soon as draw-in issues occur the gap must be reduced so the force can be increased further. Additionally, also wider gaps should be tested.
This Agile Testing Procedure clearly reduces the number of samples by extending the experimental range. Simultaneously, a good overview of the manufacturing space is obtained. Hence, the Agile Testing Procedure is optimal for performing screening or feasibility trials. It is not intended to conduct a whole formulation development or validation steps. To leverage all benefits from the Agile Testing Procedure for roller compaction, a state-of-the-art roller compactor and good knowledge about the dry granulation process are necessary.
The main reasons to use Agile Testing Procedures for first experiments in roller compaction in an overview:
- Minimum use of material
- Reduced numbers of samples
- Less effort to evaluate the variations
- Less manpower
- Faster development
- Extended experimental range
- Ideal performing screening or feasibility trials
- No physical limits due to draw-in issues

About the author:
Barbara Fretter, PhD, is co-founder and managing director of Solids Development Consult GmbH, Leverkusen, Germany. Academic background as post-doc at the University of Bonn and years of experience have turned Barbara into a true specialist for formulation development and roller compaction. Her expertise makes her a knowledgeable troubleshooter and instructor for most solid dosage issues.
Follow Barbara Fretter and Solids Development on LinkedIn or sign up for our newsletter.
With Expertise From Solids Development:
We Plan All Your Manufacturing Steps
In our laboratories we offer you our support in the field of Solid Dosage Forms. With our competence in formulation development and manufacturing processes, we overcome the gap between Pharma and Technology. We analyze, develop, and optimize your specific projects – and assume the preparation for scale-up. You want to know more about our techniques and equipment? Get in touch with us!

